Overview
This is a 5-day, in-person workshop for those looking to broaden their perspectives encompassing design thinking, product development, regulatory, intellectual property, clinical trial planning, business planning and market access.
Learning Outcomes
Biodesign Bootcamp: Deep Dive Into HealthTech Innovation with an End in Mind is jointly presented by SGInnovate and Singapore Biodesign. This workshop will help health & medtech innovators to internalise and apply the validated Biodesign framework of Identify, Invent and Implement towards structuring any new health and medtech innovation projects from a needs-centric and holistic view with an eye towards commercialization and adoption. Course Objectives By the end of the workshop, you will be able to achieve the following enabling learning outcomes according to the relevant innovation skill and competency from the innovation training whitepaper listed below: Product Development Competency (Basic) - Demonstrate ability to apply the Biodesign Framework to unmet healthcare/clinical needs and design a needs specification document based on focused research and stakeholder engagement - Apply ideation strategies to brainstorm possible healthcare solutions and where it would meet gaps in the patient/user journey and choose and screen based on a fundamental understanding of IP, regulatory, business and reimbursement models - Define and prepare prototyping plan and early risk assessment - Assess technology risks and develop functional proof-of-concept prototypes, and determine what tests are required to de-risk the project - Collaborate with different internal stakeholders (e.g., engineers and scientists) to define product requirements, develop prototype and validate results Regulatory Competency (Basic) - Conduct preliminary research to determine the regulatory class of a healthcare product as a medical device, drug, combination or IVD IP Competency (Basic) - Explain basic IP anatomy, types of IPs, IP regulations, IP lifecycle, costing and types of IP instruments - Apply principles of patentability and freedom to operate - Prepare a preliminary prior art search to evaluate healthcare innovation idea to inform feasibility Business Planning Competency (Basic) - Prepare a preliminary market analysis (top-down and bottom up) - Apply basic presentation and pitching proficiency - Have an overview of fundraising and investment landscape (i.e., public and private funding sources and options Clinical Trial Competency (Basic) - Apply basics of clinical trial planning: overview, pros and cons of study designs (e.g., RCT), clinical trial ethics guidelines (e.g., HBRA/IRB), timeline of different clinical trial stages, basic statistics concept, and implication of clinical endpoints used Market Access Competency (Basic) - Apply principles of coding, coverage and payment
Recommended Prerequisites
Entry Requirements - Bachelor's Degree or equivalent
Schedule
Date: 31 Jul 2023, Monday
Time: 9:00 AM - 5:30 PM (GMT +8:00) Kuala Lumpur, Singapore
Location: TBC, To be confirmed
Date: 01 Aug 2023, Tuesday
Time: 9:00 AM - 5:30 PM (GMT +8:00) Kuala Lumpur, Singapore
Location: TBC, To be confirmed
Date: 02 Aug 2023, Wednesday
Time: 9:00 AM - 5:30 PM (GMT +8:00) Kuala Lumpur, Singapore
Location: TBC, To be confirmed
Date: 03 Aug 2023, Thursday
Time: 9:00 AM - 5:30 PM (GMT +8:00) Kuala Lumpur, Singapore
Location: TBC, To be confirmed
Date: 04 Aug 2023, Friday
Time: 9:00 AM - 5:30 PM (GMT +8:00) Kuala Lumpur, Singapore
Location: TBC, To be confirmed
Agenda
| Day/Time | Agenda Activity/Description |
|---|---|
| 31 Jul | - Opening and Introduction - Overview of workshop - Researching on Needs - Filling up of DSTM worksheet - Need Criteria - Concept Generation / Initial Concept Screening - Brainstorm! - Overview of Concept Screening - Concept Screening worksheets - Debrief |
| 1 Aug | - Needs presentation - Introduction to DSTM template - Asian Case Study - Need Research - Preparation for Need Specification exercise - Need review and development of need criteria - Sharing of Need Criteria - Concept Generation and Initial Concept Selection - Concept development and debrief |
| 2 Aug | - Review of Day 2 and Opening - Concept sharing on what was developed in Day 2 - IP Fundamentals - IP in Asia - IP Exercise - Regulatory Fundamentals - Regulatory Affairs in Asia - Regulatory Exercise - Business models innovation in Asia - Business models exercise - Medtech Innovation in Asia: Validating Needs and concepts |
| 3 Aug | - Concept Exploration and Testing - Prototype Planning - Presentation of prototype - Reimbursement Fundamentals - Market Access to China - Final Concept Selection - Recap and Next Steps - R&D Strategy, Design Control - Clinical trials fundamentals - Case studies of clinical trials |
| 4 Aug | - Ops Plan and Financial Modeling - Implementation in Asia - Funding landscape - Preparation for presentation - Presentations - Fireside Chat - Closing |
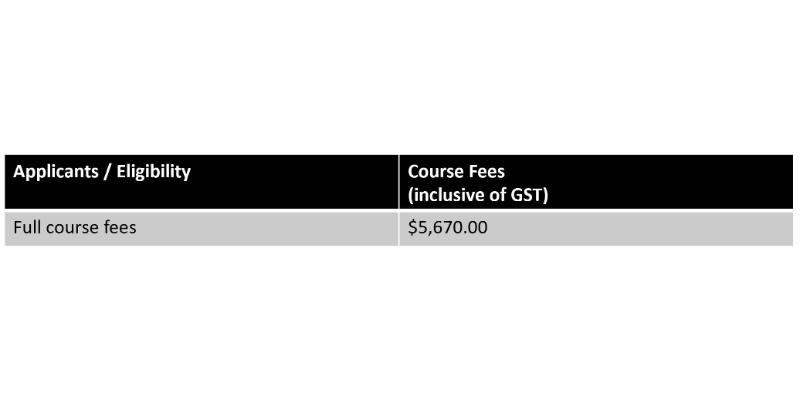
Pricing