Overview
Health products cover a wide spectrum of modalities, ranging from pharmaceuticals, biologics to medical technology. The regulatory frameworks and requirements differ between medical technology and the others.
This course explores the regulatory frameworks, clinical evaluation processes, and risk management strategies essential for the development and approval of medical devices. It provides a detailed understanding of preclinical testing, clinical evidence generation, and post-market surveillance, highlighting best practices for ensuring device safety and performance.
Course Description & Learning Outcomes
Describe the regulatory concepts and major frameworks governing the development and regulatory management of medical devices and technology
List the key regulatory guidance as well as requirements for regulatory submission and compliance of medical devices
Articulate the important considerations for assessing the clinical performance of medical devices and technology
Schedule
End Date: 07 Aug 2026, Friday
Monday - Friday, 830 AM - 530 PM
Location: Duke-NUS Medical School, 8 College Rd, Singapore 169857, 169857Pricing
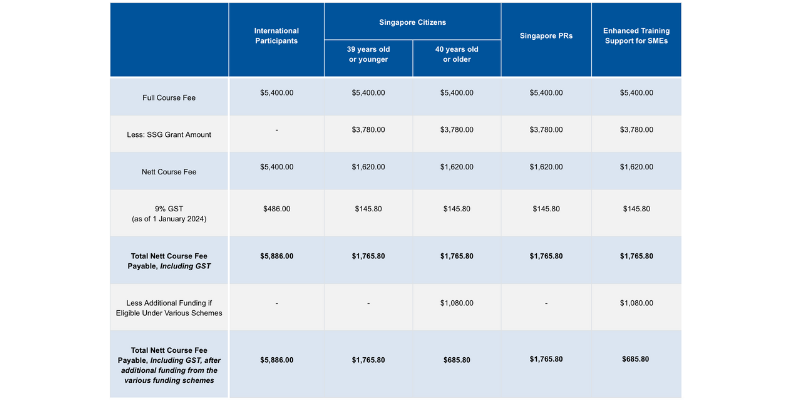
Course fees: SGD
Skills Covered
PROFICIENCY LEVEL GUIDE
Beginner: Introduce the subject matter without the need to have any prerequisites.
Proficient: Requires learners to have prior knowledge of the subject.
Expert: Involves advanced and more complex understanding of the subject.
- Product Development (Proficiency level: Beginner)






