Overview
Post market vigilance is a key regulatory function in the total product life cycle. Continual monitoring and reporting of medical device adverse events is critical in ensuring the marketed devices are free from unacceptable risk.
This course will introduce you to the key activities and roles critical for effective post-market vigilance. You will gain insights into managing adverse events, implementing field safety corrective actions, and navigating change management processes.
Course Description & Learning Outcomes
Describe the post-market regulatory requirements of medical devices
Explain the activities involved in Adverse events and Field safety corrective action
Describe the benefit-risk assessment, root cause analysis and Corrective Action Preventive Action (CAPA)
Explain key regulatory considerations in product changes from safety issues
List harmonised guidance documents related to post-market vigilance of medical devices
Schedule
End Date: 13 Mar 2026, Friday
Location: Duke-NUS Medical School, 8 College Rd, Singapore 169857
Pricing
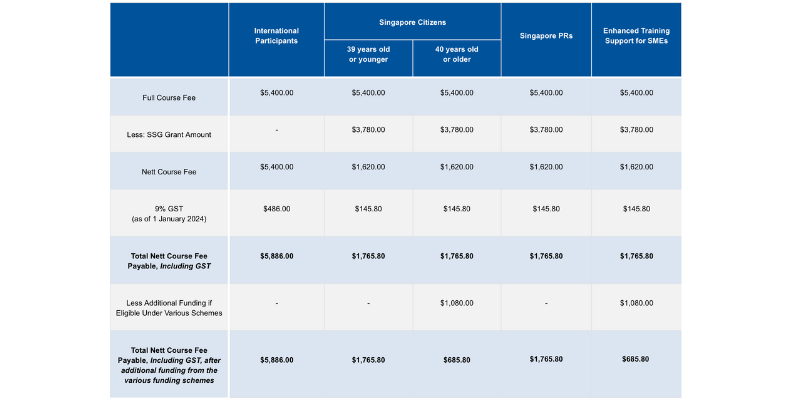
Course fees: SGD
Skills Covered
PROFICIENCY LEVEL GUIDE
Beginner: Introduce the subject matter without the need to have any prerequisites.
Proficient: Requires learners to have prior knowledge of the subject.
Expert: Involves advanced and more complex understanding of the subject.
- Product Management (Proficiency level: Proficient)
- Quality assurance (QA) (Proficiency level: Proficient)






