Overview
The effective regulation of pharmaceuticals and medical devices for safety and efficacy depends on the availability, understanding and appropriate implementation of relevant guidelines and the processes designed to ensure quality in decision-making. The requirements are frequently different from traditional clinical trials and specific to regulatory affairs.
This course will provide you with an understanding of the unique requirements for clinical trials and clinical data that support regulatory evaluation and approvals. You will learn how to navigate the processes involved in ensuring clinical data meets the necessary standards for regulatory success.
Course Description & Learning Outcomes
Explain the design of various phases of clinical trials, along with the regulatory requirements necessary to support market approvals of pharmaceutical products
Understand the regulatory requirements and processes for submitting marketing authorisation applications, as well as assessing the benefit-risk profile of pharmaceuticals through a structured framework
Recognise the impact of regulatory controls on the product life cycle and their role in maintaining the quality, safety and effectiveness of products
Schedule
End Date: 06 Feb 2026, Friday
Location: Academia, SGH Campus, 20 College Rd, Singapore 169856
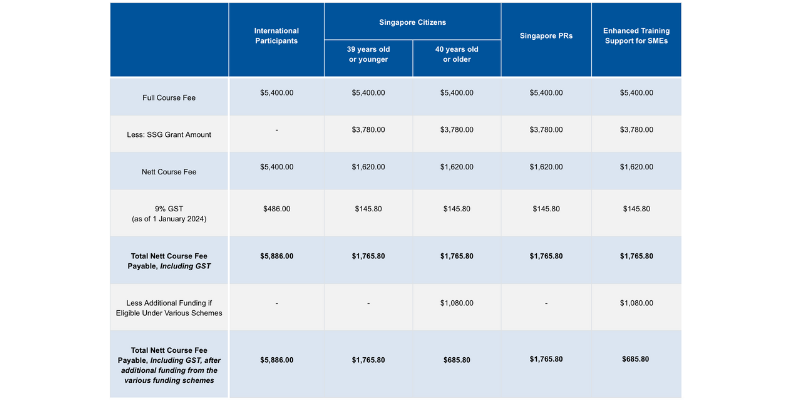
Pricing
Course fees: SGD
Skills Covered
PROFICIENCY LEVEL GUIDE
Beginner: Introduce the subject matter without the need to have any prerequisites.
Proficient: Requires learners to have prior knowledge of the subject.
Expert: Involves advanced and more complex understanding of the subject.
- Product Development (Proficiency level: Proficient)






