Overview
Ensuring the quality of pharmaceuticals is a key regulatory responsibility, for industry, manufacturers and regulators. The role of Chemistry, Manufacturing and Controls (CMC) in the successful control of pharmaceutical quality spans from product development, manufacture, process validation to post-market variation changes, as well as an optimal quality management system.
This course provides the foundation in understanding the regulatory science behind the development, manufacturing and control of pharmaceuticals, including the global guidances that shapes the regulatory processes. Besides promoting good submissions and evaluation practices, the course aims to enhance regulatory convergence and cooperation on CMC regulation.
Course Description & Learning Outcomes
Explain the importance of manufacturing and quality control for pharmaceuticals.
Learn about the strict controls and regulatory requirements, particularly on product stability and specifications, in addition to the practical skills obtained through mock reviews of dossier materials.
Understand the utility of systems approach to quality control, and the regulatory trends upcoming in this domain
Schedule
End Date: 16 Oct 2026, Friday
Monday - Friday, 830 AM - 530 PM
Location: Duke-NUS Medical School, 8 College Rd, Singapore 169857, 169857Pricing
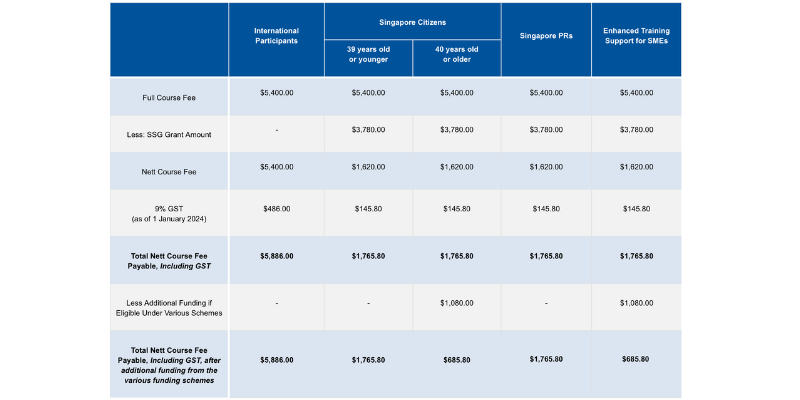
Course fees: SGD
Skills Covered
PROFICIENCY LEVEL GUIDE
Beginner: Introduce the subject matter without the need to have any prerequisites.
Proficient: Requires learners to have prior knowledge of the subject.
Expert: Involves advanced and more complex understanding of the subject.
- Product Management (Proficiency level: Beginner)
- Quality assurance (QA) (Proficiency level: Beginner)






